Semaglutide Rybelsus, Ozempic, NN9535, OG217SC, NNC 0113-0217 Ad investigationes tantum usus
Semaglutide, sub notam nomina vendiditOzempic,WegovyetRybelsus, estantidiabetic medicamentousus est ad curatiotypus 2 diabeteet ut-anti dolore dolore magna aliquaad longum tempuspondus procuratio, developed byNovo Nordiskanno MMXII. Semaglutide est aGLP-1 receptor agonistid est, humanam imitantem actionemincretin glucagon-sicut peptide-1(GLP-1), eo quod augeturinsulinsecretionem et augendaesanguis sugararbitrio et improvingglycemic imperium.Partis effectus sunt nausea, vomitus, diarrhoea, dolor abdominis, et constipatio. Mense Decembri 2017, versio injectabilis nomine Ozempic approbata est.Mense Septembri 2019, versio quae per os (Rybelsus) probata est, et mense Iunio 2021, iniectio superior-dosis sub notam nomine Wegovy vendita est, pro longo tempore ponderis administratione in adultis ab US approbata est.Cibus et medicamentis Administration(FDA).Mense Ianuario anni 2023, FDA dedit Novo Nordisk licentiam retractandi pittacium ad indicandum Rybelsum oralem uti posse.prima linea curatiopro adultis cum speciebus 2 diabete, id est in hominibus qui aliud diabete medicamentum non prius sumpserunt. Anno 2020, semaglutides erat 129th in Civitatibus Foederatis Americae maxime medicamen praescriptum, cum plus quam 4 decies centena praescriptiones. Synonyma: Rybelsus, Ozempic, NN9535, OG217SC, Ad investigationes tantum usus.
Actio biologica
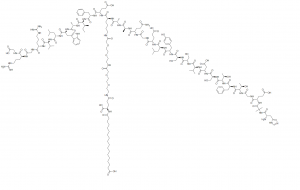
| Descriptio | Semaglutide (Rybelsus, Ozempic, NN9535, OG217SC, NNC 0113-0217), glucagonum peptidum 1 (GLP-1) analogum longum est.GLP-1 receptoragonist cum potentia ad tractationem generis diabete mellitus (T2DM). |
| scuta | GLP-1 receptor |
| In vitro | Semaglutide electa est ut meliorem candidatum semel weekly.Semaglutides duas habet substitutiones amino acido GLP-1 humano comparatas (Aib8, Arg34) et derivatur ad lysinam 26. Affinitas GLP-1R semaglutidis (0.38 ± 0,06 nM) triplex minuitur cum liraglutide, albumin autem affinitas. augetur. |
| In vivo | Plasma semivivum 46.1 h in mini-porcis sequentis iv administrationis, et semaglutides MRT of 63.6 h post sc dosing ad porcos minios habet. |
Protocol (a reference)
| Investigationes cellae: | Cell lines:BHK cellulae Concentus:0.01 pM - 0.1 μM Incubatio tempus:3 h Methodus:Congelata cellularum BHK aliquot, quae tam hGLP-1R quam CRE ignitae luciferas (clone FCW467-12A/KZ10-1) dilapsae sunt, bis in PBS lavantur, et in quiddam primordium suspenduntur.Cellulae deauratae sunt in laminas 96-bene cum 5000 cellulis/tam in volumine 50 µL.Composita exploranda diluuntur in quiddam primordium et 50 µL aliquot transfertur ad bracteam continens cellulas ut ad concentrationes finales de 1 10 perveniant.-141 10-7M. Catillus incubatus pro 3 h ad 5% CO230 °C.Patella in cella temperie pro 15 min ante permissum est ut 100 μL de stabilili plus reagens addito.Patina operitur ad protegendum illud a luce et agitata ad locus temperatus per 30 min.Patella legitur in instrumento NXT TopCount. |
Solubilitas (25°C)
| In vitroBatch: | DMSO | 3 mg/mL(0.73 mM) |
| Ethanol | Insolubilis | |
| Aquae | Insolubilis |
Informationes chemica
| M. Pondus | 4113.58 | ||
| Formulae | C187H291N45O59 | ||
| CAS Non. | 910463-68-2 | ||
| Repono | III annos | -20°C | pulvis |
| Annis II | -80°C | in solvendo | |
| naviportans | Locus temperatus shipping(Quality de gratis: productum est bonum 37℃ pro saltem 1 octo). | ||
Orci Trial Information
| NCT Number | Recruiting | Interventus | Conditiones | Sponsor/Collaborators | Satus Date | Phases |
| NCT05537233 | Nondum recruiting | Drug: Semaglutide|Drug: Placebo | Typus 1 Diabetes|OBESITY | University of Colorado Denver|Juvenile Diabetes Research Foundation | Ianuarii 1 2023 | Phase 2 |
| NCT04885634 | Nondum recruiting | Drug: Semaglutide Injectable Product|Drug: Placebo | Atrial Fibrillation | AUCTARIUM et ADIPS | Axel Brandes Herlev and Gentofte Hospital | Octobris 2022 | Phase 3 |
| NCT05579977 | recruiting | Drug: PF-07081532|Alia: Placebo|Drug: Rybelsus | Diabetes Mellitus | | Pfizer | die 27 Octobris 2022 | Phase 2 |
| NCT05254314 | recruiting | Medicamento: Semaglutide Pen Injector 2.4mg weekly|Alia: Placebo | Suspiriosis | Vanderbilt University Medical Centre|National Institute of Allergy and Infectious Diseases (NIAID) | Septembris 7 2022 | Phase 2 |
| NCT05478252 | recruiting | Drug: Semaglutide J|Drug: Semaglutide B | Diabetes Mellitus Type 2 | Novo Nordisk A/S | Augusti 3 2022 | Phase 3 |
(Notitia ex *https://clinicaltrials.gov, updated in 2022-11-29)